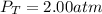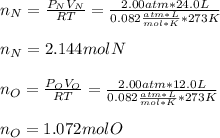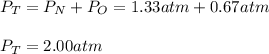In a chemistry laboratory, a student filled a 10.0 L container with two (2) different gases. The
gases are nitrogen gas taken from 24.0 L container at 2.00 atm and 12.0 L container of
oxygen at 2.00 atm. If the temperature of the gases is 273 K, calculate the partial pressure
of both gases in the resulting mixture and the total pressure.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 01:30, Michael845313
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
In a chemistry laboratory, a student filled a 10.0 L container with two (2) different gases. The
ga...
Questions in other subjects:


History, 09.04.2021 05:50


Engineering, 09.04.2021 05:50

Computers and Technology, 09.04.2021 05:50

Mathematics, 09.04.2021 05:50



Physics, 09.04.2021 05:50

History, 09.04.2021 05:50