
Chemistry, 13.10.2020 14:01 chancecharles9oug353
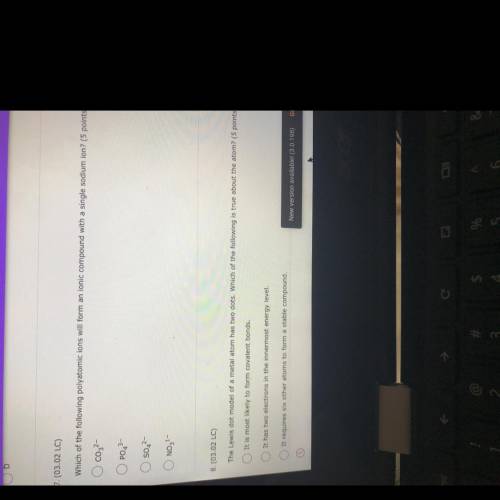
Which of the following polyatomic ions will form an ionic compound with a single sodium ion?
co₂2-
PO4^3-
S04^2-
NO₃²-

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Which of the following polyatomic ions will form an ionic compound with a single sodium ion?
co₂2-<...
Questions in other subjects:

History, 15.01.2021 08:50

Mathematics, 15.01.2021 08:50

English, 15.01.2021 08:50

Mathematics, 15.01.2021 08:50








