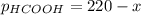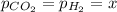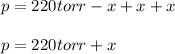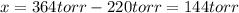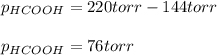Chemistry, 13.10.2020 03:01 91miketaylor
When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay: HCOOH(g) →CO2(g) + H2 (g) The rate of reaction is monitored by measuring the total pressure in the reaction container. Time (s) . . . P (torr) 0 . . . . . . . . . 220 50 . . . . . . . . 324 100 . . . . . . . 379 150 . . . . . . . 408 200 . . . . . . . 423 250 . . . . . . . 431 300 . . . . . . . 435 At the start of the reaction (time = 0), only formic acid is present. What is the formic acid pressure (in torr) when the total pressure is 364? Hint: use Dalton's law of partial pressure and the reaction stoichiometry.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3



Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay: HCO...
Questions in other subjects:



Mathematics, 02.03.2021 20:00



Biology, 02.03.2021 20:00


English, 02.03.2021 20:00

English, 02.03.2021 20:00

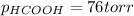
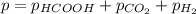
 as the time goes by:
as the time goes by: