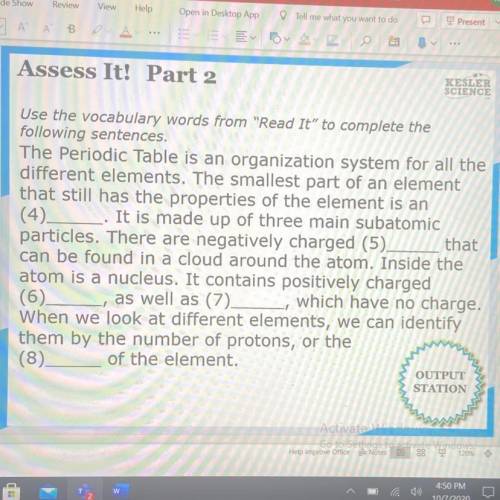
NEED THIS DONE ASAP HELP A GIRL OUT!
...

Chemistry, 12.10.2020 23:01 apexdude2020
NEED THIS DONE ASAP HELP A GIRL OUT!

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3


Chemistry, 23.06.2019 15:30, rociomartinez9
In most resting cells, the concentration of sodium ions is higher outside of cells compared with the intracellular fluid. when cells are stimulated, sodium ion channels open, and sodium diffuses from the outside of the cell to the inside of the cell. sodium ion concentrations in a resting cell are an example of and sodium ion movement in a stimulated cell is an example of in most resting cells, the concentration of sodium ions is higher outside of cells compared with the intracellular fluid. when cells are stimulated, sodium ion channels open, and sodium diffuses from the outside of the cell to the inside of the cell. sodium ion concentrations in a resting cell are an example of and sodium ion movement in a stimulated cell is an example of potential energy; kinetic energy kinetic energy; potential energy the energy of motion; stored energy chemical work; energy stored in chemical bonds
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 02.04.2021 01:40




Mathematics, 02.04.2021 01:40



Mathematics, 02.04.2021 01:40




