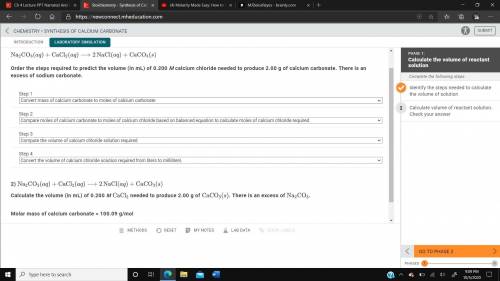
Calculate the volume of 0.200 m cacl2 needed to produce 2.00 g of caco
...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, penny3109
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2
You know the right answer?
Questions in other subjects:

Chemistry, 25.03.2021 23:20


Mathematics, 25.03.2021 23:20

Physics, 25.03.2021 23:20

Mathematics, 25.03.2021 23:20



Mathematics, 25.03.2021 23:20