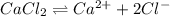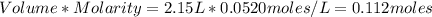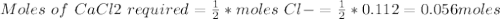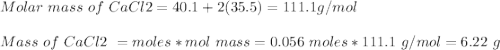Chemistry, 23.08.2019 23:20 maggiemae4993
An analytical procedure requires a solution of chloride ions. how many grams of cacl2 must be dissolved to make 2.15 l of 0.0520 m cl-? [atomic mass: ca=40.1 g/mol; cl=35.5 g/mol]

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2



Chemistry, 22.06.2019 15:30, christopherluckey7
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
An analytical procedure requires a solution of chloride ions. how many grams of cacl2 must be dissol...
Questions in other subjects:

Biology, 03.09.2021 21:10



History, 03.09.2021 21:10

Computers and Technology, 03.09.2021 21:10

Mathematics, 03.09.2021 21:10

Mathematics, 03.09.2021 21:10