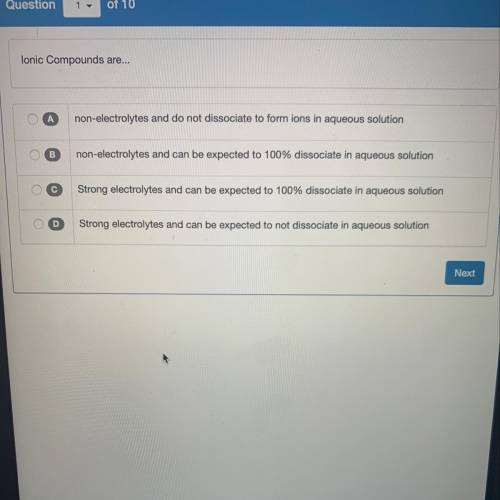
Lonic Compounds are...
A
non-electrolytes and do not dissociate to form ions in aqueous solut...

Chemistry, 10.10.2020 16:01 sbelgirl2000
Lonic Compounds are...
A
non-electrolytes and do not dissociate to form ions in aqueous solution
B
non-electrolytes and can be expected to 100% dissociate in aqueous solution
с
Strong electrolytes and can be expected to 100% dissociate in aqueous solution
D
Strong electrolytes and can be expected to not dissociate in aqueous solution
Next

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 29.01.2020 23:58

Social Studies, 29.01.2020 23:58

History, 29.01.2020 23:58

Mathematics, 29.01.2020 23:58

Mathematics, 29.01.2020 23:58




History, 29.01.2020 23:58




