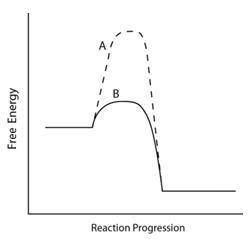
Consider the energy diagram below.
Which line indicates a higher reaction rate?
A because it...

Chemistry, 09.10.2020 19:01 gaberamos973
Consider the energy diagram below.
Which line indicates a higher reaction rate?
A because it has a lower activation energy.
B because it has a lower activation energy.
A because its (angle)Grxn is much lower.
B because its (angle)Grxn is much lower.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Questions in other subjects:

Arts, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40

Mathematics, 15.12.2020 20:40

Advanced Placement (AP), 15.12.2020 20:40

Mathematics, 15.12.2020 20:40





Chemistry, 15.12.2020 20:40



