Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2HgO(s)Hg(l) + O2(g)
At...

Chemistry, 08.10.2020 14:01 PresleyPie2700
Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2HgO(s)Hg(l) + O2(g)
At a certain temperature, a chemist finds that a reaction vessel containing a mixture of mercury(II) oxide, mercury, and oxygen at equilibrium has the following composition:
compound amount
HgO 24.0g
Hg 23.6g
O2 22.7g
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, ashleyjaslin
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
Questions in other subjects:

Biology, 12.02.2021 03:10

History, 12.02.2021 03:10

Social Studies, 12.02.2021 03:10

English, 12.02.2021 03:10

Mathematics, 12.02.2021 03:10

Mathematics, 12.02.2021 03:10

Health, 12.02.2021 03:10

Chemistry, 12.02.2021 03:10

Chemistry, 12.02.2021 03:10

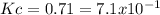
![Kc=[O_2]](/tpl/images/0794/3421/af0f4.png)
![[O_2]=\frac{22.7g*\frac{1mol}{32g} }{1L} =0.709M](/tpl/images/0794/3421/9d34c.png)


