Chemistry, 08.10.2020 14:01 justijust500
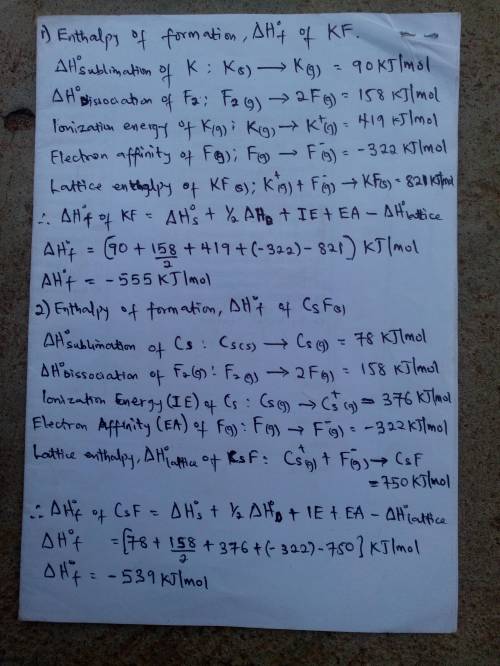
Calculate the enthalpies of formation, ΔHf∘, of the group 1 fluoride compounds from their elements using the Born–Haber cycle.
Process H∘, /
sublimation of K(s) 90
sublimation of Cs(s) 78
dissociation of F2(g) 158
ionization energy of K(g) 419
ionization energy of Cs(g) 376
electron affinity of F(g) −322
lattice enthalpy of KF(s) 821
lattice enthalpy of CsF(s) 750

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Calculate the enthalpies of formation, ΔHf∘, of the group 1 fluoride compounds from their elements u...
Questions in other subjects:

Mathematics, 22.01.2020 01:31

English, 22.01.2020 01:31



Mathematics, 22.01.2020 01:31




English, 22.01.2020 01:31

Mathematics, 22.01.2020 01:31