Chemistry, 08.10.2020 07:01 musiclyhollywoodbabo
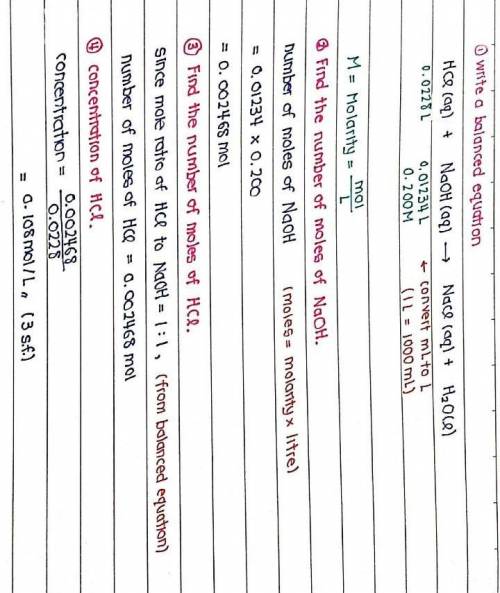
The titration of 22.80 mL of HCl solution of unknown concentration requires 12.34 mL of a 0.200 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution in M? Express your answer in moles per liter to three significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 23.06.2019 00:30, quintink
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
The titration of 22.80 mL of HCl solution of unknown concentration requires 12.34 mL of a 0.200 M Na...
Questions in other subjects:



Mathematics, 03.06.2021 20:00


Mathematics, 03.06.2021 20:00


English, 03.06.2021 20:00


English, 03.06.2021 20:00