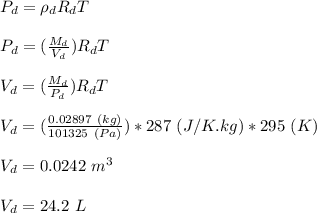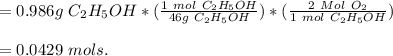Chemistry, 08.10.2020 03:01 diegomacias2411
A student wants to burn a 1.25 mL sample of ethanol (C2H5OH, d = 0.789 g/mL) in a jar containing dry air. Assuming the air in the jar is at standard atmospheric pressure and room temperature (22 °C), what volume will the jar need to be in order to hold enough oxygen for complete combustion? Hint: Refer to the composition of dry air in the previous question. (a) Write a balanced chemical reaction for the combustion of ethanol. (b) Calculate the moles of oxygen needed to completely combust the ethanol. (c) Calculate the partial pressure of oxygen in the jar. (d) Calculate the volume of oxygen (in L) needed in t

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, paolaviviana
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1


Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3
You know the right answer?
A student wants to burn a 1.25 mL sample of ethanol (C2H5OH, d = 0.789 g/mL) in a jar containing dry...
Questions in other subjects:

Biology, 15.07.2019 01:30

Mathematics, 15.07.2019 01:30

Mathematics, 15.07.2019 01:30


Mathematics, 15.07.2019 01:30




Mathematics, 15.07.2019 01:30