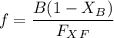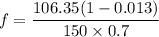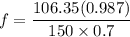Chemistry, 08.10.2020 01:01 joannakawata6
A distillation column is separating 150.0 kmol/h of a saturated liquid mixture that is 30.0 mol% methanol and 70.0 mol% water. The column operates at 1.0 atm pressure. Reflux ratio is 2.0, and reflux is returned as a saturated liquid. We desire a 97.0% recovery of methanol in the distillate and a methanol distillate mole fraction of 0.990. Find distillate ow rate D, bottoms flow rate B, methanol mole fraction in the bottoms xM, bot, and the fractional recovery of water in the bottoms.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1


Chemistry, 23.06.2019 06:40, donalyndearingbizz
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
A distillation column is separating 150.0 kmol/h of a saturated liquid mixture that is 30.0 mol% met...
Questions in other subjects:

Arts, 22.01.2021 17:40

Biology, 22.01.2021 17:40

History, 22.01.2021 17:40


Mathematics, 22.01.2021 17:40

Mathematics, 22.01.2021 17:40



Mathematics, 22.01.2021 17:40

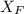 = 30%
= 30%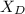 = 0.990
= 0.990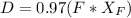
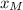 can be computed by using the formula:
can be computed by using the formula: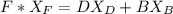
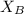 )
)