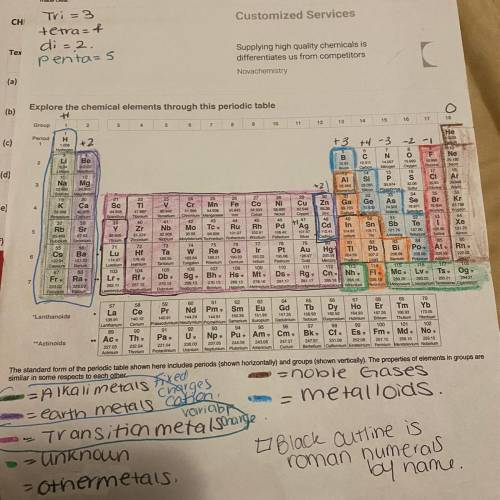
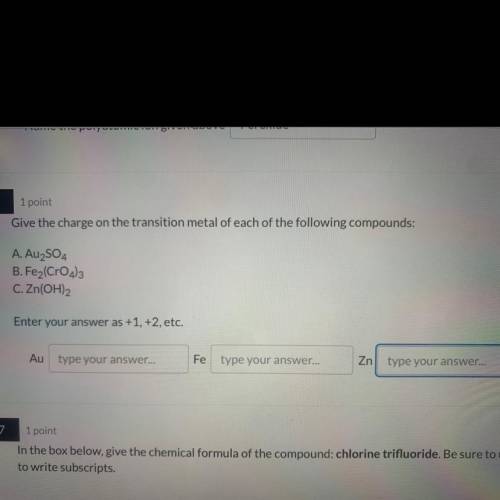
Give the charge on the transition metal of each of the following compounds:
A. Au2SO4
B. Fe2(...

Chemistry, 05.10.2020 15:01 joneskc0629
Give the charge on the transition metal of each of the following compounds:
A. Au2SO4
B. Fe2(CrO4)3
C. Zn(OH)2
Enter your answer as +1, +2, etc.
Au
type your answer...
Fe
type your answer...
Zn
type your answer...

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 13:30, citlalli30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 20.05.2020 05:57

History, 20.05.2020 05:57

History, 20.05.2020 05:57


Social Studies, 20.05.2020 05:57

Mathematics, 20.05.2020 05:57



Biology, 20.05.2020 05:57

Spanish, 20.05.2020 05:57