
Terry bought a gold necklace at a pawnshop and was wondering if it was “real” gold. She decided to perform a density test. She placed the necklace in a graduated cylinder of water that had 47.00 mL of water and the water rose to 48.35 mL. She then weighed the necklace and found it to have a mass of 22.89 g. What is the density of Terry’s necklace?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1


Chemistry, 22.06.2019 02:40, alexandraparava
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 08:00, kleathers97
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3
You know the right answer?
Terry bought a gold necklace at a pawnshop and was wondering if it was “real” gold. She decided to p...
Questions in other subjects:








Social Studies, 03.03.2022 05:30

Arts, 03.03.2022 05:30

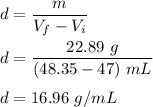 '
'

