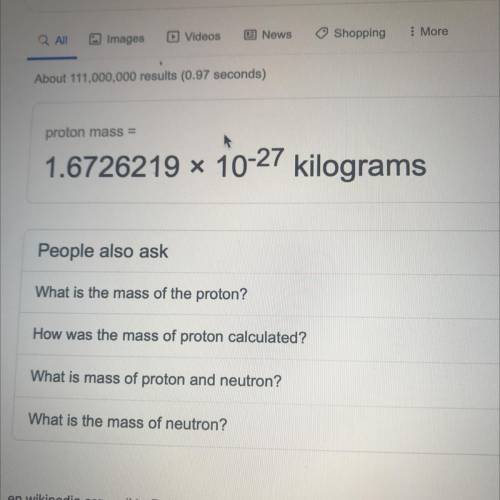
The mass of a proton is
A: substantially greater than the mass of a neutron.
B: roughly...


Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

You know the right answer?
Questions in other subjects:




Mathematics, 01.06.2021 16:50

Mathematics, 01.06.2021 16:50


German, 01.06.2021 16:50



Advanced Placement (AP), 01.06.2021 16:50