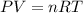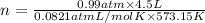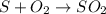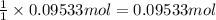Chemistry, 14.10.2019 21:40 shardonnay2160
What mass of sulfur has to burn to produce 4.5l so2 at 300°c and 101 kpa in the following reaction?
a. 13.5 g s
b. 3.07 g s
c. 68.8 g s
d. 41.0 g s

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3


Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
What mass of sulfur has to burn to produce 4.5l so2 at 300°c and 101 kpa in the following reaction?...
Questions in other subjects:


Mathematics, 06.03.2021 19:20



English, 06.03.2021 19:20





History, 06.03.2021 19:20