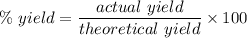Chemistry, 02.10.2020 21:01 briannahernand2
Compound A reacts with Compound B to form only one product, Compound C, and it's known the usual percent yield of C in this reaction is 40%. Suppose 10.0 g of A are reacted with excess Compound B, and 6.4 g of Compound C are successfully isolated at the end of the reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
Compound A reacts with Compound B to form only one product, Compound C, and it's known the usual per...
Questions in other subjects:


Mathematics, 12.08.2020 04:01


Social Studies, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01


Mathematics, 12.08.2020 04:01


Mathematics, 12.08.2020 04:01

Mathematics, 12.08.2020 04:01