Chemistry, 02.10.2020 19:01 jtswagg6634
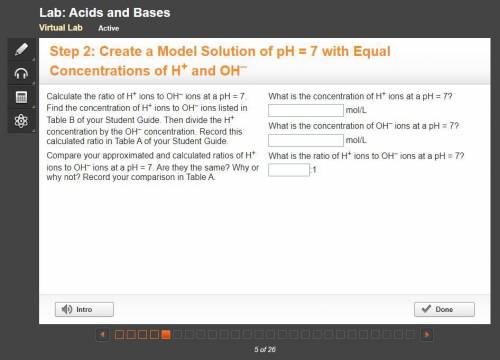
10 PTS FOR CORRECT ANSWER Calculate the ratio of H+ ions to OH– ions at a pH = 7. Find the concentration of H+ ions to OH– ions listed in Table B of your Student Guide. Then divide the H+ concentration by the OH– concentration. Record this calculated ratio in Table A of your Student Guide.
Compare your approximated and calculated ratios of H+ ions to OH– ions at a pH = 7. Are they the same? Why or why not? Record your comparison in Table A.
What is the concentration of H+ ions at a pH = 7?
mol/L
What is the concentration of OH– ions at a pH = 7?
mol/L
What is the ratio of H+ ions to OH– ions at a pH = 7?
:1

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
10 PTS FOR CORRECT ANSWER Calculate the ratio of H+ ions to OH– ions at a pH = 7. Find the concentra...
Questions in other subjects:





Mathematics, 13.10.2019 00:00

Mathematics, 13.10.2019 00:00


Social Studies, 13.10.2019 00:00


Chemistry, 13.10.2019 00:00