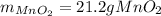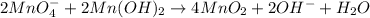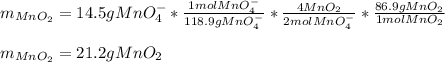Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, aricketts3901
How does chemistry affect our world? a. chemicals makes our world more polluted. b. chemicals keeps us healthy. c. chemicals can or hurt our world. d. chemicals make our world safe to live in.
Answers: 1

Chemistry, 21.06.2019 22:00, breannaasmith1122
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3
You know the right answer?
If 14.5 g of MnO4- (permanganate) react with manganese (II) hydroxide how many grams of manganese (I...
Questions in other subjects:

Mathematics, 15.10.2019 14:30

Business, 15.10.2019 14:30



Physics, 15.10.2019 14:30

Mathematics, 15.10.2019 14:30

Mathematics, 15.10.2019 14:30

History, 15.10.2019 14:30


Physics, 15.10.2019 14:30