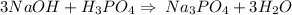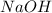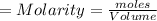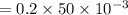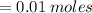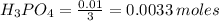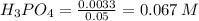Chemistry, 02.10.2020 15:01 mayamcmillan11
PLZ HELP CHEMISTRY! WILL REWARD! Please correct me if I'm wrong.
I marked my answers as x.
1. During a titration, 50.0 ml of 0.2M NaOH were required to neutralize 50.0ml of H3PO4. What's the concentration of the H3PO4 solution?
A. 1.8M
B. 0.6M
C. 0.07M
x D. 0.2M
2. Attached photo - Using the two cell reduction potentials shown for their corresponding reaction, calculate the cell potential for a voltaic cell made from these two systems.
A. –1.68 V
B. –0.78 V
C. 0.78 V
D. 1.68 V
3. If atoms from two different elements react to form a compound, the element with a higher will have a negative oxidation number.
A. atomic number
B. atomic radius
C. energy
x D. electronegativity
4. During the electrolysis of an aqueous solution of sodium nitrate, a gas forms at the anode, what gas is it?
x A. Oxygen
B. Hydrogen
C. Sodium
D. Water
5. The lithium-ion in a lithium-ion battery moves from one side to the other by
A. moving through the cathode.
B. moving through the anode.
x C. passing through the separator.
D. passing through the device.
6. The use of water in a heat exchanger is taking advantage of water's
x A. high specific heat.
B. significant expansion during boiling.
C. solid state when frozen.
D. polar molecular nature.
Which of the following goes through the largest volumetric change?
A. Water when it boils into steam
x B. Water when it freezes into ice
C. Water when it's heated from 1oC to 99oC
D. Ice when it melts into water

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 05:50, carog24
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2
You know the right answer?
PLZ HELP CHEMISTRY! WILL REWARD! Please correct me if I'm wrong.
I marked my answers as x.
Questions in other subjects:


English, 04.08.2019 19:30



Spanish, 04.08.2019 19:30

Mathematics, 04.08.2019 19:30




Social Studies, 04.08.2019 19:30

 is the right choice.
is the right choice.