
Chemistry, 02.10.2020 09:01 Slytherinsarethebest
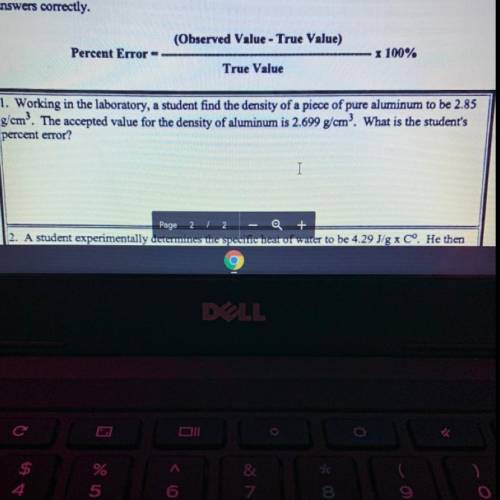
1. Working in the laboratory, a student find the density of a piece of pure aluminum to be 2.85
g'om? The accepted value for the density of aluminum is 2.699 g/cm? What is the student's
percent error?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, genyjoannerubiera
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 23.06.2019 00:00, juliannasl
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 01:00, MrTeriffic
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
1. Working in the laboratory, a student find the density of a piece of pure aluminum to be 2.85
g'o...
Questions in other subjects:

English, 14.12.2021 22:50







Mathematics, 14.12.2021 22:50

Geography, 14.12.2021 22:50

Health, 14.12.2021 22:50




