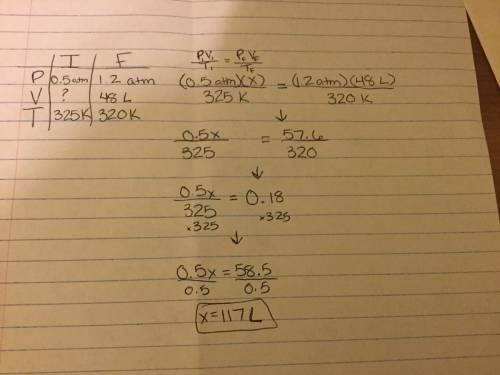Chemistry, 24.09.2019 04:30 tdluong157
An unknown volume of gas has a pressure of 0.50 atm and temperature of 325k. if the pressure is raised to 1.2atm and the temperature decreased to 320k giving a new volume of 48l what was the initial volume

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 23.06.2019 03:00, kuehlthau03
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
An unknown volume of gas has a pressure of 0.50 atm and temperature of 325k. if the pressure is rais...
Questions in other subjects:



Mathematics, 17.10.2019 11:00

History, 17.10.2019 11:00

English, 17.10.2019 11:00

Mathematics, 17.10.2019 11:00