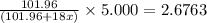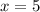Chemistry, 25.09.2020 06:01 witchhunt666
A sample of pure alumina hydrate was obtained. A 5.000 g sample of the material was heated carefully in a vacuum oven until no more mass was lost from the sample. After heating, the final weight of the material was 2.6763 g. What was the formula of the hydrated alumina, Al2O3•xH2O? (Enter a whole number for "x") (mol. wt. Al2O3 = 101.96)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, jaejaeJae9534
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2


You know the right answer?
A sample of pure alumina hydrate was obtained. A 5.000 g sample of the material was heated carefully...
Questions in other subjects:


Mathematics, 27.07.2019 03:00




Spanish, 27.07.2019 03:00




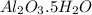

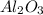 = 101.96 g/mol
= 101.96 g/mol
 decomposes to give 101.96 g of
decomposes to give 101.96 g of 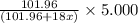 of H_2O
of H_2O