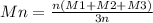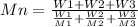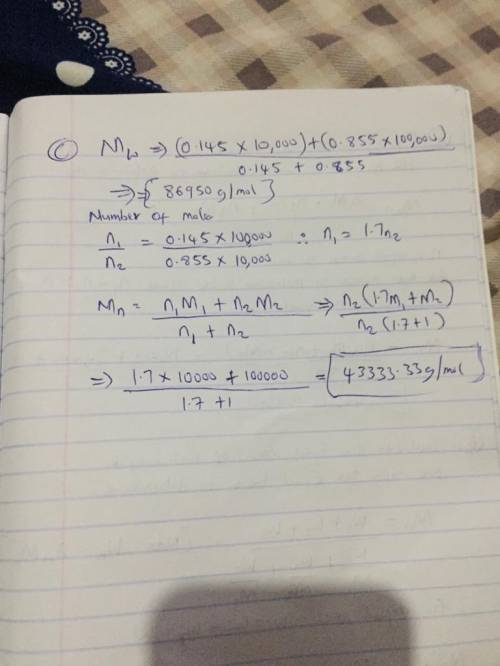Chemistry, 24.09.2020 22:01 savagesquid4807
Two mixtures were prepared from three very narrow molar mass distribution polystyrene samples with molar masses of 10,000, 30,000 and 100,000 g/mola indicated below: (A) Equal numbers of molecules of each sample (B) Equal masses of each sample. For each of the mixtures, calculate the number-average and weight-average molar masses and comment upon the meaning of the values.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1


You know the right answer?
Two mixtures were prepared from three very narrow molar mass distribution polystyrene samples with m...
Questions in other subjects:

Chemistry, 15.08.2020 18:01




Computers and Technology, 15.08.2020 18:01