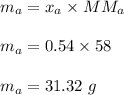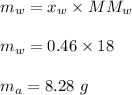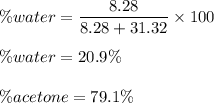Chemistry, 24.09.2020 21:01 schoolboyq3017
A mixture of water and acetone at 756 mm boils at 70.0°C. The vapor pressure of acetone is
1.54 atm at 70.0°C, while the vapor pressure of water is 0.312 atm at the same temperature.
Calculate the percentage composition of the mixture

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
A mixture of water and acetone at 756 mm boils at 70.0°C. The vapor pressure of acetone is
1.54 atm...
Questions in other subjects:

Social Studies, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

English, 18.10.2020 14:01


English, 18.10.2020 14:01

English, 18.10.2020 14:01

 ......( 1 )
......( 1 )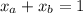 ......( 2 )
......( 2 )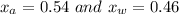 .
.