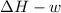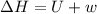Chemistry, 24.09.2020 21:01 MalikaJones
One mole of liquid water at 100°C is heated until the liquid is converted entirely to vapor at 100°C and 1 bar pressure. Calculate q, w, U, and H for each of the following:
A. The vaporization is carried out in a cylinder where the external pressure on the piston is maintained at 1 bar throughout.
B. The cylinder is first expanded against a vacuum (Pex=0) to the same volumn as in part (a), and then sufficient heat is added to vaporize the liquid completely to 1 atm pressure.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1


Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
One mole of liquid water at 100°C is heated until the liquid is converted entirely to vapor at 100°C...
Questions in other subjects:

Biology, 01.05.2021 19:50

Health, 01.05.2021 19:50




Physics, 01.05.2021 19:50



English, 01.05.2021 19:50

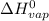 = 2260 kJ/kg
= 2260 kJ/kg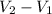 )
)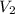 = specific volume of steam = 0.0305 m³/mol
= specific volume of steam = 0.0305 m³/mol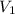 = specific volume of water = 0.00001837 m³/mol
= specific volume of water = 0.00001837 m³/mol 3.05 kJ
3.05 kJ