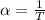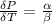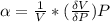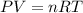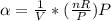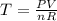The coefficient of thermal expansion α = (1/V)(∂V/∂T)p. Using the equation of state, compute the value of α for an ideal gas. The coefficient of compressibility β is define by β = -(1/V)(∂V/∂p)T. Compute the value of β for an ideal gas. For an ideal gas, express the derivative (∂p/∂T)v in terms of α and β. Do the same derivative for van der Waals gas.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
You know the right answer?
The coefficient of thermal expansion α = (1/V)(∂V/∂T)p. Using the equation of state, compute the val...
Questions in other subjects:

Mathematics, 02.07.2019 03:30


Mathematics, 02.07.2019 03:30

Mathematics, 02.07.2019 03:30





History, 02.07.2019 03:30

English, 02.07.2019 03:30