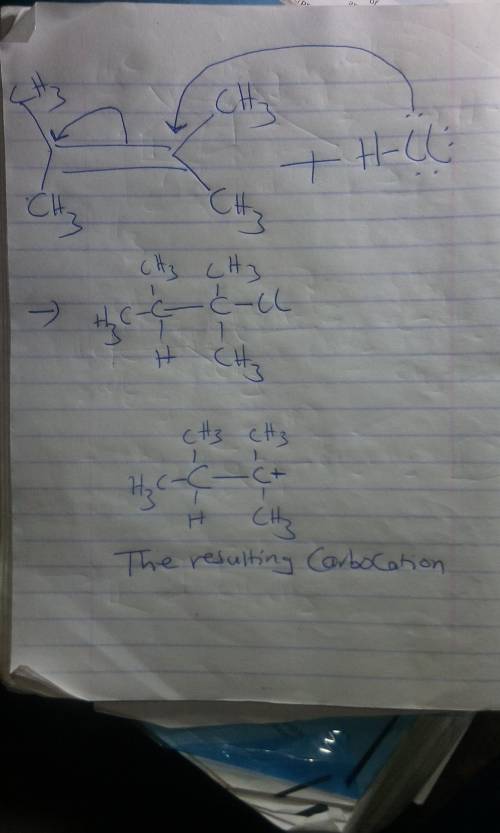
Chemistry, 24.09.2020 01:01 zaratayyibah
Double bonds can act as Lewis bases, sharing their electrons with Lewis acids.
A. Use curved arrows to show how the alkene below will react with HCl.
B. Make the ends of your arrows specify the origin and destination of reorganizing electrons.
C. Draw the resulting carbocation.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2


Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Double bonds can act as Lewis bases, sharing their electrons with Lewis acids.
A. Use curved arrows...
Questions in other subjects:


Chemistry, 10.12.2020 20:20



Mathematics, 10.12.2020 20:20


Mathematics, 10.12.2020 20:20

Mathematics, 10.12.2020 20:20

Advanced Placement (AP), 10.12.2020 20:20