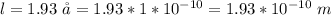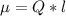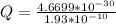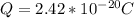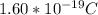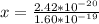Chemistry, 23.09.2020 14:01 solikhalifeoy3j1r
Determine the partial negative charge on the bromine atom in a c−br bond. the bond length is 1.93 å and the bond dipole moment is 1.40 d . express your answer using 3 significant figures. the partial negative charge on the bromine atom = previous answersrequest answer incorrect; try again; 4 attempts remaining provide feedback.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1
You know the right answer?
Determine the partial negative charge on the bromine atom in a c−br bond. the bond length is 1.93 å...
Questions in other subjects:

Mathematics, 16.02.2022 18:20



History, 16.02.2022 18:30


Chemistry, 16.02.2022 18:30

Mathematics, 16.02.2022 18:30



Law, 16.02.2022 18:30