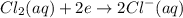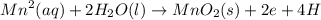Chemistry, 23.09.2020 08:01 jhenderson2024
A galvanic cell is powered by the following redox reaction: (aq) (aq) (aq)(s) (l) (l) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. Round your answer to decimal places.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1


Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
A galvanic cell is powered by the following redox reaction: (aq) (aq) (aq)(s) (l) (l) Answer the fol...
Questions in other subjects:

Mathematics, 20.11.2020 23:50

Mathematics, 20.11.2020 23:50

Chemistry, 20.11.2020 23:50

Mathematics, 20.11.2020 23:50

Mathematics, 20.11.2020 23:50

Mathematics, 20.11.2020 23:50

English, 20.11.2020 23:50

English, 20.11.2020 23:50