
Chemistry, 23.09.2020 01:01 haydenamrhein1693
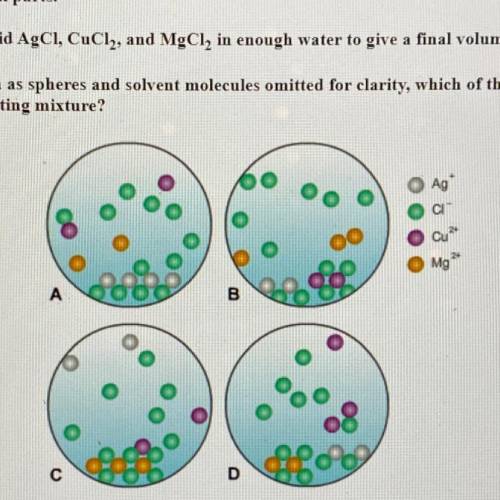
A chemist mixes solid AgCl, CuCl2, and MgCl2, in enough water to give a final volume of 50.0 mL.
(a) With ions shown as spheres and solvent molecules omitted for clarity, which of the following best
represents the resulting mixture?
(b) If each sphere represents 5.5*10^-3 mol of ions, what is the total concentration of dissolved (separated) ions?
(c) What is the total mass of the solid?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
A chemist mixes solid AgCl, CuCl2, and MgCl2, in enough water to give a final volume of 50.0 mL.
(a...
Questions in other subjects:

Mathematics, 09.03.2021 01:20

History, 09.03.2021 01:20

World Languages, 09.03.2021 01:20

Mathematics, 09.03.2021 01:20


Mathematics, 09.03.2021 01:20

Mathematics, 09.03.2021 01:20

History, 09.03.2021 01:20

Mathematics, 09.03.2021 01:20



