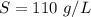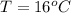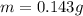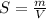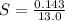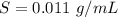Chemistry, 22.09.2020 21:01 ncontreras06
A geochemist in the field takes a 13.0 mL sample of water from a rock pool lined with crystals of a certain mineral compound X. He notes the temperature of the pool, 16.° C, and caps the sample carefully. Back in the lab, the geochemist filters the sample and then evaporates all the water under vacuum. Crystals of X are left behind. The researcher washes, dries and weighs the crystals. They weigh 0.143 g
Required:
Using only the information above, can you calculate the solubility of X in water at 15°C ? If you said yes, calculate it.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

You know the right answer?
A geochemist in the field takes a 13.0 mL sample of water from a rock pool lined with crystals of a...
Questions in other subjects:


Mathematics, 30.03.2021 02:00



History, 30.03.2021 02:00

Mathematics, 30.03.2021 02:00



English, 30.03.2021 02:00

Mathematics, 30.03.2021 02:00