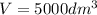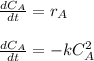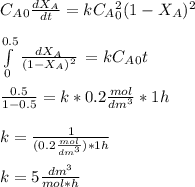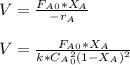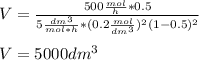Chemistry, 22.09.2020 15:01 hiyagirllyric
The elementary gas-phase reaction2A → Bis carried out in a constant-volume batch reactor where 50% conversion is achieved in 1 hour. Pure Ais charged to the reactor at an initial concentration of 0.2 mol/dm3. If the same reaction is carried outin a CSTR, what volume would be necessary to achieve 50% conversion for a feed molar flow rate of500 mol/h and an entering concentration of A of 0.2 mol/dm3? (Ans.:V = 5,000 dm3

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
The elementary gas-phase reaction2A → Bis carried out in a constant-volume batch reactor where 50% c...
Questions in other subjects:

Mathematics, 15.04.2021 20:40

Mathematics, 15.04.2021 20:40

Arts, 15.04.2021 20:40




Arts, 15.04.2021 20:40

English, 15.04.2021 20:40