
Chemistry, 20.09.2020 19:01 lucyamine0
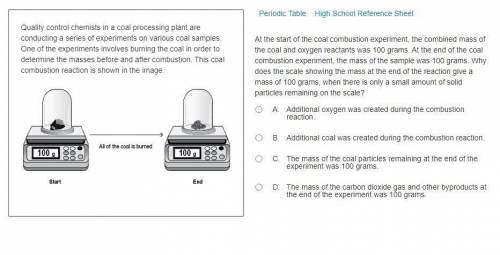
At the start of the coal combustion experiment, the combined mass of the coal and oxygen reactants was 100 grams. At the end of the coal combustion experiment, the mass of the sample was 100 grams. Why does the scale showing the mass at the end of the reaction give a mass of 100 grams, when there is only a small amount of solid particles remaining on the scale?
A. Additional oxygen was created during the combustion reaction.
B. Additional coal was created during the combustion reaction.
C. The mass of the coal particles remaining at the end of the experiment was 100 grams.
D. The mass of the carbon dioxide gas and other byproducts at the end of the experiment was 100 grams.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 23.06.2019 04:00, onegirl435
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1
You know the right answer?
At the start of the coal combustion experiment, the combined mass of the coal and oxygen reactants w...
Questions in other subjects:




English, 10.05.2021 22:50

Mathematics, 10.05.2021 22:50


English, 10.05.2021 22:50

Biology, 10.05.2021 22:50

History, 10.05.2021 22:50

Mathematics, 10.05.2021 22:50



