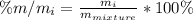Chemistry, 20.09.2020 18:01 kaylaelaine18
A student was provided with a solid sample made up of a mixture of components. Prior to separation, the student measured 2.895 g of the mixture. After separation, the student found the mixture contained the following four components:
Component 1: 1.12g
Component 2: 0.756g
Component 3: 0.254g
Component 4: 0.525g
Which component has the highest mass % for the mixture?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, grayfaith16
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 21.06.2019 17:40, monkey2865
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3
You know the right answer?
A student was provided with a solid sample made up of a mixture of components. Prior to separation,...
Questions in other subjects:




Mathematics, 07.04.2020 17:32


Mathematics, 07.04.2020 17:32

Mathematics, 07.04.2020 17:32

History, 07.04.2020 17:32


Chemistry, 07.04.2020 17:32