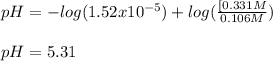A 1.41 L buffer solution consists of 0.149 M butanoic acid and 0.288 M sodium butanoate. Calculate the pH of the solution following the addition of 0.061 moles of NaOH . Assume that any contribution of the NaOH to the volume of the solution is negligible. The Ka of butanoic acid is 1.52×10−5 .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, haybaby312oxdjli
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
A 1.41 L buffer solution consists of 0.149 M butanoic acid and 0.288 M sodium butanoate. Calculate t...
Questions in other subjects:

Mathematics, 13.11.2020 04:00


English, 13.11.2020 04:00

Mathematics, 13.11.2020 04:00

Mathematics, 13.11.2020 04:00


Mathematics, 13.11.2020 04:00

Social Studies, 13.11.2020 04:00

English, 13.11.2020 04:00

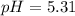
![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/0771/3232/33848.png)
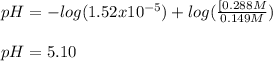
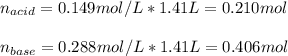
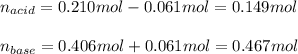
![[acid]=\frac{0.149mol}{1.41L} =0.106M](/tpl/images/0771/3232/f69f4.png)
![[base]=\frac{0.467mol}{1.41L} =0.331M](/tpl/images/0771/3232/b5538.png)