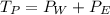Suppose that the mixture in problem 4 is at 15 OC, where the pure vapor pressures are 12.5 mmHg for water and 32.1 mmHg for ethanol. According to Raoult’s Law, the pressure of a component in a solution is equal to its pure vapor pressure times its mole fraction, that is PA = ( ) (XA). Use Raoult’s law to determine the vapor pressure of each component in the solution. Then, add them to find the total vapor pressure. Use significant figures. Show all equations and conversion factors.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, asims13
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
Suppose that the mixture in problem 4 is at 15 OC, where the pure vapor pressures are 12.5 mmHg for...
Questions in other subjects:




Mathematics, 24.03.2021 01:00

Mathematics, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00



 of water = 12.5 mmHg
of water = 12.5 mmHg