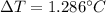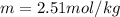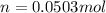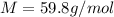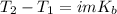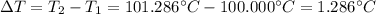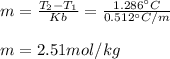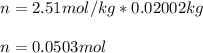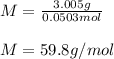Chemistry, 20.09.2020 15:01 bradleydb222
Suppose 3.005 g of a nonvolatile solute is added to 20.02 g of water (the solvent), and the boiling point increases from 100.000 OC to 101.286 OC. Determine the TB, molality, moles, and molecular weight for the solute if kb for water is 0.512 OC/m. Report each value using the correct number of significant digits. Refer to Example 1.2 and pages 3-4 in the chapter 1 notes for general chemistry 1 to understand significant figures. Also, include all applicable units and conversion factors.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, angelicar1160
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1
You know the right answer?
Suppose 3.005 g of a nonvolatile solute is added to 20.02 g of water (the solvent), and the boiling...
Questions in other subjects:

Social Studies, 03.11.2020 18:20

English, 03.11.2020 18:20

Biology, 03.11.2020 18:20


Biology, 03.11.2020 18:20

Social Studies, 03.11.2020 18:20


Mathematics, 03.11.2020 18:20

English, 03.11.2020 18:20

Biology, 03.11.2020 18:20