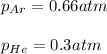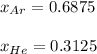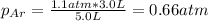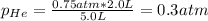Chemistry, 20.09.2020 15:01 noeliaortiz3478
Two containers, one with a volume of 3.0 L and the other with a volume of 2.0 L contain, respectively, argon gas at 1.1 atm and helium at 0.75 atm. The containers are initially separated by a valve, and then the valve is opened to connect the two containers. Assume perfect gases and determine the followings.
a. The total pressure of the mixed gases
b. The partial pressure of each gas
c. The mole fraction of each gas

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, lizethdominguez037
What is the value of keq for the reaction expressed in scientific notation
Answers: 1


You know the right answer?
Two containers, one with a volume of 3.0 L and the other with a volume of 2.0 L contain, respectivel...
Questions in other subjects:

History, 17.09.2019 10:30


History, 17.09.2019 10:30

History, 17.09.2019 10:30




Mathematics, 17.09.2019 10:30

Mathematics, 17.09.2019 10:30

Mathematics, 17.09.2019 10:30

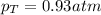 .
.