
Chemistry, 20.09.2020 15:01 smithsavannah295
olecular iodine, I2(g), dissociates into iodine atoms at 625 K with a first-order rate constant of 0.271 s-1. (a) What is the half-life for this reaction? s (b) If you start with 0.048 M I2 at this temperature, how much will remain after 5.37 s assuming that the iodine atoms do not recombine to form I2? M

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3


Chemistry, 23.06.2019 01:00, Angelofpink1143
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
olecular iodine, I2(g), dissociates into iodine atoms at 625 K with a first-order rate constant of 0...
Questions in other subjects:

Mathematics, 21.02.2021 08:50

Chemistry, 21.02.2021 08:50

Mathematics, 21.02.2021 08:50

History, 21.02.2021 08:50



Mathematics, 21.02.2021 08:50

Advanced Placement (AP), 21.02.2021 08:50


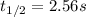
![[I_2]=0.011M](/tpl/images/0771/9802/19391.png)
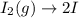
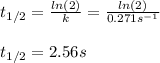
![[I_2]=[I_2]_0exp(-kt)](/tpl/images/0771/9802/e9e96.png)
![[I_2]=0.048Mexp(-0.271s^{-1}*5.37s)\\\\](/tpl/images/0771/9802/4a6e2.png)


