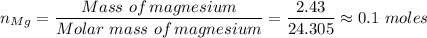Chemistry, 20.09.2020 16:01 msdmdsm1186
You react 2.43 grams of magnesium with oxygen from the air according to the following reaction. The final mass of the product, magnesium oxide, is 4.12 grams. How many grams of oxygen reacted? 2Mg + O2 → 2MgO

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 13:30, Pranav2004
What is one measurement needed to calculate the speed of an object?
Answers: 1
You know the right answer?
You react 2.43 grams of magnesium with oxygen from the air according to the following reaction. The...
Questions in other subjects:




Mathematics, 21.05.2021 05:20

Mathematics, 21.05.2021 05:20

Mathematics, 21.05.2021 05:20




 , is given as follows;
, is given as follows;