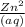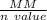or the solution containing Zn2+(aq), n is 2, so the MM/n ratio will be the molar mass of Zn divided by 2: MM/n = (65.39 g/mol) / 2 = 32.70 g/mol Compare this theoretical value with your experimental result. If the plating efficiency is significantly less than 1, say it is 0.55, how would the observed MM/n ratio compare to the theoretical value?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1


Chemistry, 23.06.2019 01:30, Nakiahalogn4
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
or the solution containing Zn2+(aq), n is 2, so the MM/n ratio will be the molar mass of Zn divided...
Questions in other subjects:

Social Studies, 13.10.2019 22:20

History, 13.10.2019 22:20

Biology, 13.10.2019 22:20


Social Studies, 13.10.2019 22:20

Business, 13.10.2019 22:20

Spanish, 13.10.2019 22:20


Mathematics, 13.10.2019 22:20