Chemistry, 20.09.2020 14:01 janessa0804
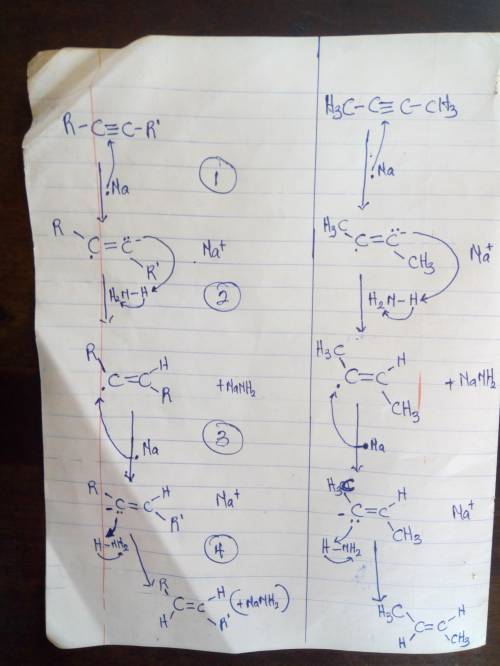
Alkynes are reduced to trans alkenes by a process called dissolving metal reduction. The reaction uses sodium or lithium metal as the reducing agent and liquid ammonia as the solvent. The method is specific in the formation of trans alkenes from alkynes. The method involves two successive transfers of single electrons from the alkali metal to the triple bond, with abstraction of protons from the ammonia solvent. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, janayflowers042
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Alkynes are reduced to trans alkenes by a process called dissolving metal reduction. The reaction us...
Questions in other subjects:

Mathematics, 21.08.2019 05:30

Biology, 21.08.2019 05:30

Health, 21.08.2019 05:30

Mathematics, 21.08.2019 05:30





History, 21.08.2019 05:30

Physics, 21.08.2019 05:30