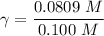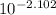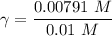Chemistry, 20.09.2020 18:01 jamesgraham577
A. The measured pH of a 0.100 M HCl solution at 25 degrees Celsius is 1.092. From this information, calculate the activity coefficient of H+.B. The measured pH of a solution of 0.010 HCl and 0.090 KCl at 25 degree Celsius is 2.102. Calculate the activity coefficient of H+ in this solution. C. Why does the pH change in part B relative to that in part A?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3


Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
A. The measured pH of a 0.100 M HCl solution at 25 degrees Celsius is 1.092. From this information,...
Questions in other subjects:

Chemistry, 09.12.2020 09:50


Biology, 09.12.2020 09:50


English, 09.12.2020 09:50

English, 09.12.2020 09:50

Law, 09.12.2020 09:50

English, 09.12.2020 09:50


Mathematics, 09.12.2020 09:50

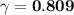
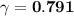
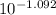
 × C
× C![\gamma = \dfrac{[a]}{C}](/tpl/images/0773/2720/33e10.png)