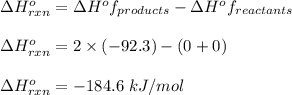Chemistry, 19.09.2020 01:01 emileehogan
Hydrogen gas, H2, reacts explosively with gaseous chlorine, Cl2, to form hydrogen chloride, HCl(g). What is the enthalpy change for the reaction of 2 mole of H2(g) with 2 mole of Cl2(g) if both the reactants and products are at standard state conditions? The standard enthalpy of formation of HCl(g) is −92.3 kJ/mol. H2(g)+Cl2(g)→2HCl(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3


Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Hydrogen gas, H2, reacts explosively with gaseous chlorine, Cl2, to form hydrogen chloride, HCl(g)....
Questions in other subjects:



Mathematics, 25.02.2020 01:52

Mathematics, 25.02.2020 01:52

Mathematics, 25.02.2020 01:52





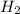 , reacts explosively with gaseous chlorine,
, reacts explosively with gaseous chlorine,  , to form hydrogen chloride, HCl(g).
, to form hydrogen chloride, HCl(g).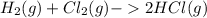
 with 2 mole of
with 2 mole of  if both the reactants and products are at standard state conditions .
if both the reactants and products are at standard state conditions .