
Chemistry, 10.09.2020 04:01 llnapier8924
A certain gas obeys the van der Waals equation with a = 0.50 m6 Pa mol−2. Its molar volume is found to be 5.00 × 10–4 m3 mol−1 at 273 K and 3.0 MPa. From this information calculate the van der Waals constant b. What is the compression factor for this gas at the prevailing temperature and pressure?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3


Chemistry, 23.06.2019 01:40, mandilynn22
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
A certain gas obeys the van der Waals equation with a = 0.50 m6 Pa mol−2. Its molar volume is found...
Questions in other subjects:


History, 31.03.2020 19:25

Social Studies, 31.03.2020 19:25




Biology, 31.03.2020 19:25



Mathematics, 31.03.2020 19:25

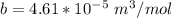

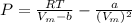
 for R , 273K for T ,
for R , 273K for T ,  for
for  ,
,  for a and
for a and  for P
for P




