
Chemistry, 10.09.2020 04:01 cookieasd9000
A 0.4066 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride ion is precipitated as AgCl by the addition of an excess of silver nitrate. The mass of the resulting AgCl is found to be 0.9260 g. What is the mass percentage of chlorine in the original compound? %

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
A 0.4066 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride...
Questions in other subjects:

Mathematics, 10.09.2020 19:01

Mathematics, 10.09.2020 19:01

English, 10.09.2020 19:01

Mathematics, 10.09.2020 19:01

Mathematics, 10.09.2020 19:01

Mathematics, 10.09.2020 19:01

Mathematics, 10.09.2020 19:01

Biology, 10.09.2020 19:01

Social Studies, 10.09.2020 19:01

Mathematics, 10.09.2020 19:01

 g of chlorine will be present in 0.9260 g of AgCl
g of chlorine will be present in 0.9260 g of AgCl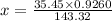
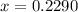 g
g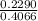 × 100%
× 100%

