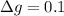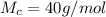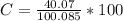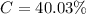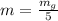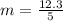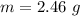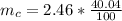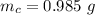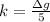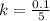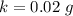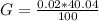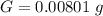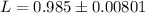Chemistry, 10.09.2020 03:01 kragland4752
g Five calcite, CaCO3 (MW 100.085 g/mol), samples of equal mass have a total mass of 12.3±0.1 g. What is the absolute uncertainty (grams) of calcium in each average calcium mass of the sample? Assume that the relative uncertainties in atomic mass are small compared the uncertainty of the total mass.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2


Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
g Five calcite, CaCO3 (MW 100.085 g/mol), samples of equal mass have a total mass of 12.3±0.1 g. Wha...
Questions in other subjects:


Mathematics, 08.10.2020 04:01

English, 08.10.2020 04:01

Mathematics, 08.10.2020 04:01





Physics, 08.10.2020 04:01

Mathematics, 08.10.2020 04:01

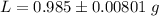
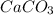 is
is