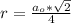The density of lead, which has the FCC structure, is 11.36 . The atomic weight of lead is 207.19 . Use Avogadro's number: 6.02210. Calculatethe lattice parameter(Enter your answer to three significant figures.) = 2.75*10^21 the atomic radius of lead(Enter your answer to three significant figures.) =

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1



Chemistry, 22.06.2019 10:50, adam1299
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments, solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
The density of lead, which has the FCC structure, is 11.36 . The atomic weight of lead is 207.19 . U...
Questions in other subjects:

Mathematics, 09.01.2020 22:31



Mathematics, 09.01.2020 22:31



History, 09.01.2020 22:31

World Languages, 09.01.2020 22:31


Spanish, 09.01.2020 22:31

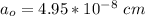


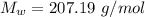


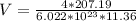

![a_o = \sqrt[3]{V }](/tpl/images/0748/9324/a210e.png)
![a_o = \sqrt[3]{1.211 5 *10^{-22} }](/tpl/images/0748/9324/32761.png)